
Regulatory Work
When operating on the industrial field of life sciences, compliance with legal and normative requirements is unavoidable.
All VERMES Medical Equipment products, which are developed and implemented for medical, pharmaceutical or biotechnological applications, follow the mandatory legal requirements.
We are happy to support you with regulatory questions to our products, eg, IVDD, MDD and GMP..

We develop systems and system components for medical diagnostics in compliance with the in-vitro diagnostic and medical device directives.
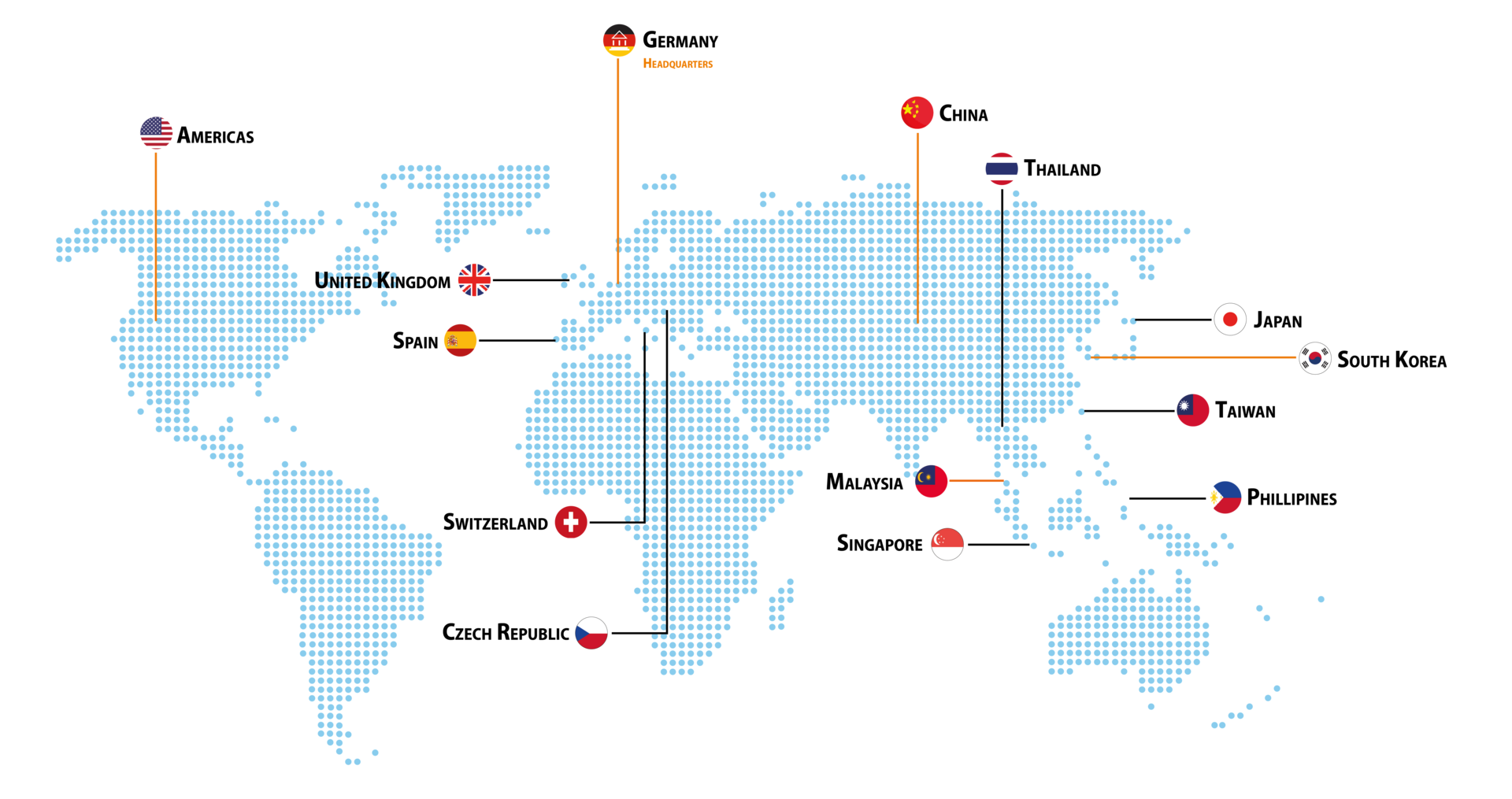
Locations
VERMES Medical Equipment has a global distribution system and supplies high tech manufacturing facilities in almost all industries.
| HEADQUARTERS | GLOBAL SUBSIDIARIES | |
|---|---|---|
VERMES Medical Equipment + 49 (0) 8024 644-0 |
+ 86 (0) 592 7257233 | . + 1 408 520-2555 |
+ 60 4 358 0996 |
+ 82 (0)32 246 1500 |

